China Viral Transport Medium Tube: Swabs & VTM Supplier
4455The 10-mix-1 virus transport medium has become an effective tool for covid-19 PCR assays because it improves the efficiency of sampling and detecti...
View detailsSearch the whole station Pandemic Supply
When sampling for nucleic acid testing, you should have seen virus transport media tubes with light red liquid or even light yellow or colourless nucleic acid sampling tubes, so what exactly is the difference between them?
Virus preservation solutions are available in clear, red, light pink and yellow, and the various colours represent different virus preservation solutions. Virus preservation solutions are also known as nucleic acid preservation solutions, sample preservation solutions, etc. It is usually divided into inactivated and non-inactivated types.
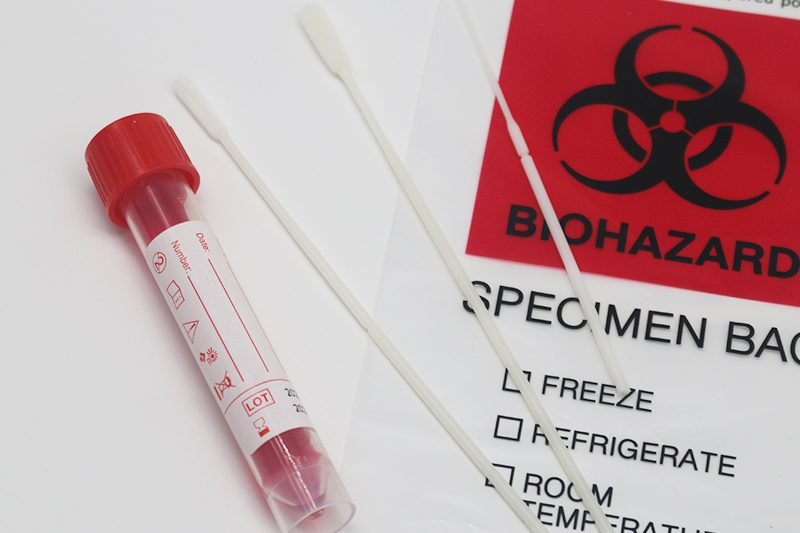
Inactivated preservation fluid is usually colourless because its composition contains no colour. If the liquid has colour, it indicates that it has added the pH indicator phenol red and caused discolouration. When the liquid is alkaline, it appears a purplish red. When the liquid is neutral, it appears red; if it is acidic, the preservation fluid will turn yellow. You can also quickly determine whether the pH of the virus preservation solution has changed or whether bacteria and viruses have grown or degraded by observing the change in colour of the liquid.
Inactivated Virus Transport Medium
Inactivated virus preservation solution is added with decomposition salt and guanidine salt, which kills the virus and inhibits bacteria, can avoid secondary infection caused by virus leakage, and has high safety.
Non-inactivating virus transport media
Non-inactivating virus preservation solution does not inactivate the virus. Still, it helps the virus maintain its intact traits and prolongs its survival time in vitro, thus improving the accuracy of nucleic acid detection, which greatly benefits experts conducting scientific research on viral nucleic acids. Since viruses cannot survive in vitro and must be parasitized inside living cells, non-inactivating virus transport media can extend the survival time of cells parasitized on viruses and indirectly add liquid components to improve virus survival.
“HCY, Health care for you” is our forever mission. We dedicate to offering safe & reliable products and medical services with our global creditable partners. HCY has already supplied to WHO, MAYO clinic, MGI, DDC, Yale University, Qorvo, Quanterix, Thomas Scientific, SD biosensor, Cardinal Health, Cleveland Clinic, Mars Petcare & LumiraDx, etc. in the past years.
The 10-mix-1 virus transport medium has become an effective tool for covid-19 PCR assays because it improves the efficiency of sampling and detecti...
View detailsThe full name of VTM is Virus Transport Medium, which is mainly used for collecting, transporting and storing biological samples.
View detailsThe red liquid in the virus transport medium is called virus preservation fluid.
View detailsOnly by preserving the collected samples in virus transport medium can the stability of the virus be maintained, thus helping the orderly execution...
View detailsWe value your privacy We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
Our Privacy Policy