10ml Viral Transport Medium (Inactivated), VTM Tube
4675The viral transport medium is designed for the inactivation of various viruses, such as 2019-ncov, influenza, and for the transportation of virus n...
View detailsSearch the whole station Pandemic Supply
Proprietary Name:Universal Transport Medium Viral Transport Media Kit(VTM Kit)
Made in china
FDA ISO13485 NMPA CE MDR
Classification Name: CULTURE MEDIA, SINGLE BIOCHEMICAL TEST
Product Code: JSF
Device Class: 1
Regulation Number: 866.2320
Medical Specialty: Microbiology
Registered Establishment Name: HUACHENYANG(SHENZHEN)TECHNOLOGY CO., LTD
Registered Establishment Number: 3011649813
Owner/Operator: Huachenyang(Shenzhen)Technology Company Ltd
Owner/Operator Number: 10048986
Establishment Operations: Foreign Exporter; Manufacturer
Universal Transport Medium is an FDA-cleared collection and transport system suitable for the collection, transport, maintenance, and long-term freeze storage of clinical specimens containing viruses, including COVID-19, chlamydia, mycoplasma or ureaplasma organisms. The transport medium comes in a plastic, screw cap tube and maintains organism viability for 48 hours at room or refrigerated temperature.
Universal Transport Medium is an FDA-cleared collection and transport system suitable for the collection, transport, maintenance, and long-term freeze storage of clinical specimens containing viruses, including COVID-19, chlamydia, mycoplasma or ureaplasma organisms. The transport medium comes in a plastic, screw cap tube and maintains organism viability for 48 hours at room or refrigerated temperature.
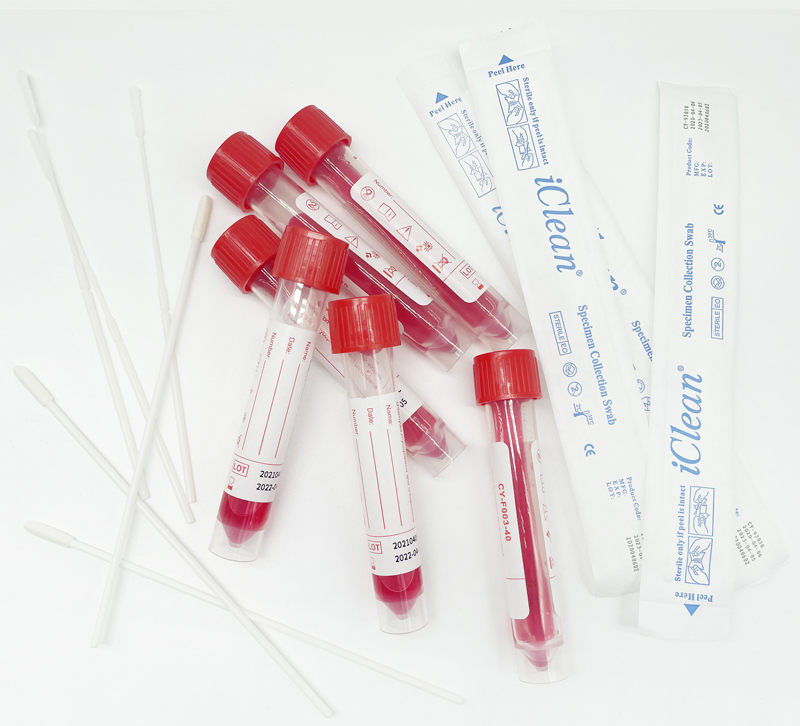
Room temperature stable system maintains the viability of organisms for up to 48 hours and does not require refrigeration storage prior to collection
Universal Transport Medium has been tested and validated in full compliance with CLSI M40-A2: Quality Control of Microbiological Transport System Standard
Universal Transport Medium has been used successfully for Rapid Antigen Testing, DFA, Viral Culture, and for Molecular-Based Assays * Unique media formulation includes antibiotics to inhibit bacterial and fungal growth, without affecting viruses, chlamydia, mycoplasma, or ureaplasma
Rapid release and dispersion of virus particles. Three glass beads in each tube facilitate the mixing and dispersion of patient sample material and release of virus particles from the swab during vortexing
A phenol red pH indicator provides a visual gauge of the medium integrity throughout the storage and life of the product
The viral transport medium is designed for the inactivation of various viruses, such as 2019-ncov, influenza, and for the transportation of virus n...
View detailsThe DNA Collection Kit includes sampling swabs and sample preservation tubes for improved usability.
View detailsNon-inactivated viral transport medium contains solution components conducive to virus cell culture, which can ensure the integrity and stability o...
View detailsDisposable virus transport medium can help medical staff collect viruses and monitor samples.
View detailsWe value your privacy We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
Our Privacy Policy