What is the liquid in the virus transport medium? Why is it red?
3162The viral transport medium(VTM) has only one mission - to keep the genetic material in the test sample intact.
View detailsSearch the whole station Pandemic Supply
COVID testing’s precision and sensitivity are crucial for controlling pandemics. Viral transport medium (VTM) only needs to be registered as a Class 1 medical device in the majority of nations, and the quality varies. Purchasers focus more on factors like price, material, and size while ignoring more important technical indicators like microbial index and limit of detection (LOD), which makes it harder to control pandemics.
VTM is a type of disposable consumable used to maintain the stability of the nucleic acids or to preserve the vitality of viral samples.
A preservation solution, known as the red liquid in VTM, is available in two varieties: inactivated and non-inactivated. The use of inactivated VTM, which can render a virus inactive, prevent cross-infection during testing, and suppress the activity of nucleases to prevent the hydrolysis of viral nucleic acids, is advised by the CDC.
Covid-19 is particularly unstable because of its genetic material made of RNA. RNA integrity is essential for accurate nucleic acid detection and it might be impaired by high temperatures or nucleases.
The key indicators for determining the quality of VTM are the preservation effect, limit of detection, microbiological index, virus inactivation efficiency, and other viral nucleic acid-related factors.
The preservation effect, limit of detection (LOD), microbial index, inactivation rate are the key indicators for determining the precision and sensitivity of COVID test.
The role of the preservation solution is to retain the integrity of viral RNA and inhabit degradation and hydrolysis. The virus is inactivated in VTM, releasing the genetic material. Single-stranded RNA is exceedingly unstable in vitro. RNA integrity is affected by temperature, transportation circumstances, and storage duration. The COVID test is more accurate the greater the RNA protection.
In order to ensure that samples with low viral loads are detected and prevent false negative miscalculations, the CDC mandates that the COVID test’s sensitivity be limited to 500 copies/ml. In actuality, samples are not sent to a lab for testing right away. Due to inappropriate storage, samples with relatively low viral loads may drop below the 500 copies/ml concentration, causing false-negative test results and missing and incorrect tests.
The total number of aerobic bacteria (CFU/mL) ≤ 200; Total number of molds and yeasts (CFU/mL) ≤ 100.
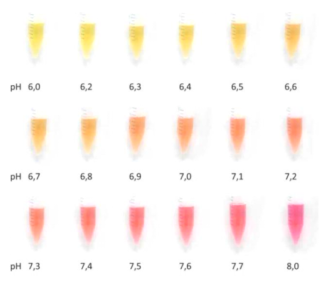
Color indicators (usually phenolphthalein or phenolic red sodium salt) are added to the preservation solution to enable visual inspection of the sample’s condition. The liquid ought to be alkaline and purple-red in hue under normal circumstances. The pH value will decrease as a result of the bacteria’ reproduction, the color will change from red to yellow, and it will become obvious that the VTM is contaminated once the microbes no longer meet the criteria. Microorganisms speed up the degradation of viral RNA, which has an adverse effect on the COVID test’s sensitivity.
In order to significantly lower the risk of cross-infection during sample collection, transportation, and experimental activities, inactivated VTMs are required on the front lines. Inactivation rate > 99% in 30 min.
In the face of enormous testing demand and market, there will unavoidably be uneven products. But in the public health sector, product quality is more important than price. Therefore we must uphold the principle of quality first to support scientific Covid-19 prevention and control.
“HCY, Health care for you” is our forever mission. We dedicate to offering safe & reliable products and medical services with our global creditable partners. HCY has already supplied to WHO, MAYO clinic, MGI, DDC, Yale University, Qorvo, Quanterix, Thomas Scientific, SD biosensor, Cardinal Health, Cleveland Clinic, Mars Petcare & LumiraDx, etc. in the past years.
The viral transport medium(VTM) has only one mission - to keep the genetic material in the test sample intact.
View detailsWhat are the benefits of scaling? What are the benefits of scaling? And does scaling hurt your teeth?
View detailsThe DNA Collection Kit includes sampling swabs and sample preservation tubes for improved usability.
View detailsSince the virus itself will quickly lyse in vitro and thus affect the results of subsequent testing, virus transport media are required to preserve...
View detailsWe value your privacy We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
Our Privacy Policy