5″ Sterile Large Round Foam Swab
45Professional custom production of various specifications of sponge cotton swabs, dust-free purification cotton swabs, purification cotton swabs are...
View detailsSearch the whole station Pandemic Supply
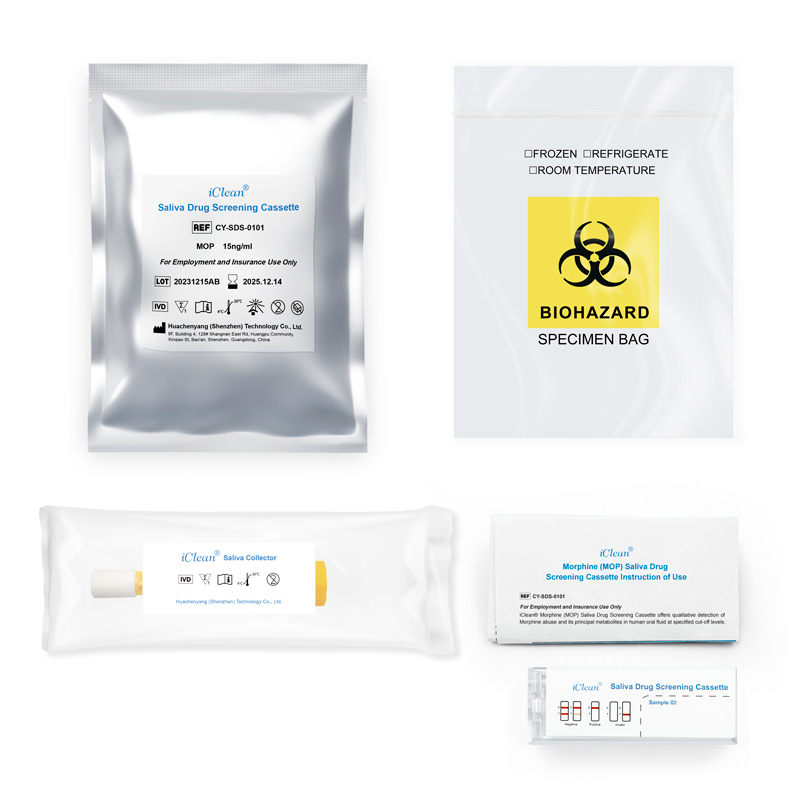
Morphine (MOP) Saliva Drug Screening Cassette
Instruction of Use
CY-SDS-0101
For Employment and Insurance Use Only
iClean® Morphine (MOP) Saliva Drug Screening Cassette offers qualitative detection of Morphine abuse and its principal metabolites in human oral fluid at specified cut-off levels.
Intended Use
The MOP Saliva Drug Screening Cassette is rapid saliva screening test. The test is a lateral flow, immunoassay for the qualitative detection of specific drug and its metabolites in human saliva at the following cut-off concentrations:
| Test | Calibrator | Cut off (ng/mL) |
| Morphine (MOP) | Morphine | 15 |
This test will detect other related compounds, please refer to the Analytical Specificity table in this package insert.
The assay provides a qualitative, preliminary test result. A more specific analytical method must be used in order to obtain a confirmed result. Gas Chromatography/Mass Spectrometry (GC/MS) or Liquid Chromatography/Tandem Mass Spectrometry (LC/MS-MS) are the preferred confirmatory methods. Professional judgment should be applied to any drug of abuse test result, particularly when preliminary positive results are indicated.
Summary
The MOP Saliva Drug Screening Cassette is a rapid saliva screening test that can be performed without the use of an instrument. The test utilizes monoclonal antibodies to selectively detect elevated levels of specific drugs in saliva.
Morphine(MOP):Morphine is a popular marketed drug (Serax) for treatment of moderate to severe pain. It is also a common metabolite of opiates [morphine, codeine (methyl-morphine), and heroin (semi-synthetic derivatives of morphine)]. The opiates are administered either by smoking, intravenous injection, intramuscular injection or oral ingestion. Adverse or toxic effects of opiates usage include papillary constriction, constipation, urinary retention, nausea, vomiting, hypothermia,drowsiness, dizziness, apathy, confusion, respiratory depression, hypotension, cold and clammy skin, coma,and pulmonary edema. Death may occur following an over dosage.
The duration of effect of morphine is 3-6 hours. Morphine is metabolized extensively, with only 2-12% excreted as unchanged morphine in the urine.Heroin is rapidly metabolized to morphine in the body; the pattern of urinary excretion of heroin is similar to that of morphine.Codeine is also extensively metabolized, 10-15% of the dose is demethylated to form morphine and norcodeine. It has been reported that the unchanged morphine may remain detectable in urine for up to one week, which make morphine a marker of opiates abuse.
Principle
During testing, a saliva specimen migrates upward by capillary action. A drug, if present in the Saliva specimen below its cut-off concentration, will not saturate the binding sites of its specific antibody. The antibody will then react with the drug-protein conjugate and a visible colored line will show up in the test region of the specific drug dipstick. The presence of drug above the cut-off concentration will saturate all the binding sites of the antibody. Therefore, the colored line will not form in the test region. A drug-positive saliva specimen will not generate a colored line in the specific test region of the dipstick because of drug competition, while a drug-negative saliva specimen will generate a line in the test region because of the absence of drug competition.
To serve as a procedural control, a colored line will always appear at the control region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
Precautions
Materials
Materials Provided
Material Required but Not Provided
Storage and Stability
1.Store at 4°C-30°C(39℉-86℉) in the sealed pouch up to the expiration date.
2.Keep away from direct sunlight, moisture and heat.
3.DO NOT FREEZE.
4.Preferably open the pouch only shortly before collection and testing.
Specimen Collection and Preparation
Test Procedure
Allow the kit and saliva specimen to reach room temperature (65°F-86°F/18ºC-30ºC) prior to testing.
AVOID PLACING ANYTHING IN THE MOUTH 10 MINUTES PRIOR TO TESTING.
Note: Make sure the sponge collector is inserted vertically and the handle of collector is put into the clamp.
Step 1 Step 2 Step 3
Interpretation of Results
Negative Positive Invalid
Negative (-)
A colored band is visible in the Control Region (C) and the appropriate Test Region (T). It indicates that the concentration of the corresponding drug of that specific test zone is zero or below the detection limit of the test.
Positive (+)
A colored band is visible in the Control Region (C). No colored band appears in the appropriate test region. It indicates a positive result for the corresponding drug of that specific Test Region (T).
Invalid
If a colored band is not visible in the Control Region (C), the test is invalid. Another test should be run to re-evaluate the specimen. If test still fails, please contact the distributor with the lot number.
Note: There is no meaning attributed to line color intensity or width.
Quality Control
Though there is an internal procedural control line in the test device of Control Region (C), the use of external controls is strongly recommended as good laboratory testing practice to confirm the test procedure and to verify proper test performance. Positive and negative control should give the expected results. When testing the positive and negative control,the same assay procedure should be adopted.
Limitations of Procedure
1.The test provides only a qualitative, preliminary result. A secondary analytical method must be used to obtain a confirmed result. Gas Chromatography/Mass Spectrometry (GC/MS) or Liquid Chromatography/Tandem Mass Spectrometry (LC/MS-MS) are preferred confirmatory methods.
2. A positive test result does not indicate the concentration of drug in the specimen or the route of administration.
3. A negative result may not necessarily indicate a drug-free specimen. Drug may be present in the specimen below the cutoff level of the assay.
Performance Characteristic
Standard drugs were spiked into negative PBS pool to the concentration of 0% Cut-off, -50% Cut-off,-25% Cut-off,Cut-off,+25% Cut-off and +50% Cut-off.The results were summarized below.
| Drug Conc.(Cut-off range) | N | MOP | |
| – | + | ||
| 0% Cut-off | 30 | 30 | 0 |
| -50% Cut-off | 30 | 30 | 0 |
| -25% Cut-off | 30 | 28 | 2 |
| Cut-off | 30 | 12 | 18 |
| 25% Cut-off | 30 | 3 | 27 |
| 50% Cut-off | 30 | 0 | 30 |
| Compound | ng/mL |
| Morphine | |
| Morphine | 15 |
| Codeine | 100 |
| Ethyl morphine | 100 |
| Hydromorphine | 1000 |
| Hydrocodone | 2000 |
| Levorphanol | 400 |
| Morphine 3-β-D-Glucuronide | 50 |
| Norcodeine | 1500 |
| Normorphine | 12500 |
| Nalorphine | 10000 |
| Oxycodone | 30000 |
| Oxymorphone | 25000 |
| Thebaine | 1500 |
Considering the complexity of clinical saliva specimens and the possibility that various saliva specimens contain potentially interfering substances, we simulated above situations by adding the potentially interfering substances to a certain concentration as specimen. The following components show no cross-reactivity when tested with iClean® MOP Saliva Drug Screening Cassette at a concentration of 100 ug/mL.
| (+/-) Chlorpheniramine | Dipehnhydramine | Magnesium |
| 4-Hydroxyphencyclidine | d-Norpropoxyphene | Maprotiline |
| Acetaminophen | Domperidone | Mega-T Plus |
| Acetylmorphine | Doxepine | Metoprolol tartrate |
| Aciclovir | d-Propoxyphene | Mifepristone |
| Afrin | Enalapril maleate | Montelukast |
| Aleve | Epinephrine HCl | Morphine |
| Alphenol | Esomeprazole | Mosapride |
| AM2201 4-Hydroxypentyl metabolite | Estroven | Narcotine |
| Amiodarone HCl | Ethyl Glucuronide | Nifedipine |
| Amitriptyline | Ethylmorphine | Nikethamide |
| AmLodipine Mesylate | Fenofibrate | Nimodipine |
| Amobarbital | Fluvoxamine | Nordoxepine |
| Amoxicillin | Fuel | Notriptyline |
| Ampicillin | Gabapentin | Omeprazole |
| Aprobarbital | Glibenclamide | Oxycodone |
| Aripiprazole | Gliclazide | Papaverine |
| Aspirin | Glipizide | PCM |
| Atorvastatin | Glucosamine | Penfluridol |
| Atropine | Glucose | Penicillin V |
| Buprenorphine | Haloperidol | Pentobarbital |
| Butabarbital | Heartburn Relief | Phencyclidine |
| Butalbital | Hydrochlorothiazide | Pheniramine |
| Butathal | Hydromorphone | Phenobarbital |
| Caffeine | I Caps | Pioglitazone HCl |
| Captopril | Imipramine | Piracetam |
| Carbamazepine | Isosorbide dinitrate | Potassium |
| Cefaclor | JWH-018 (Spice Cannabinoid) | Pravastatin |
| Cefradine | JWH-018 4-Hydroxypentyl metabolite-D5 | Promazine |
| Cephalexin | JWH-018 N-4-hydroxypentyl | Promethazine |
| Chondroitin | JWH-018 Pentanoic Acid | Propylthiouracil |
| Ciprofloxacin | JWH-019 6-hydroxypentyl | Rifampicin |
| Citrate | JWH-073 (Spice Cannabinoid) | Secobarbital |
| Clarithromycin | JWH-073 3-Hydroxybutyl metabolite | Sildenafil citrate |
| Clomipramine | JWH-073 3-Hydroxybutyl metabolite-D5 | Simvastatin |
| Clopidogrel bisulfate | JWH-073 Butanoic Acid | sodium |
| Clozapine | JWH-122 N-4-hydroxypentyl | Spironolactone |
| Codeine | JWH-210 5-Hydroxypentyl metabolite | Tetracycline |
| Cortisone | Ketoconazole | Tramadol |
| Cotinine | Levofloxacin | Trazodone HCl |
| CVS | Levonorgestrel | Triamterene |
| Cyclopentobarbital | Levothyroxine sodium | Trimipramiine |
| Desipramine | Lidocaine HCl | Vitamin B1 |
| Dextromethorphan HBr | Lisinopril | Vitamin B2 |
| Diclofenac sodium | Lithium carbonate | Vitamin C |
| Dihydrocodeine | Loratadine | Zencore Plus2 |
From the results above, it is clear that iClean® MOP Saliva Drug Screening Cassette resists well against interference from these substances.
Index of Symbols
| Temperature limit | Consult instructions for use | ||
| Do not re–use | Batch code | ||
| Contains sufficient for <n> tests | Keep away from sunlight | ||
| Use-by date | Manufacturer | ||
| Catalogue number | Do not use if package is damaged and consult instructions for use | ||
| In vitro Diagnostic medical device |
Manufacturer
Huachenyang (Shenzhen) Technology Co., Ltd.
8F, Building 4, 128# Shangnan East Rd, Huangpu Community, Xinqiao St, Bao’an, Shenzhen, Guangdong, China
Website: www.chenyanglobal.com
Tel: 86-755-27393226 Fax: 86-755-27381080
Effective Date
November 11, 2023
Professional custom production of various specifications of sponge cotton swabs, dust-free purification cotton swabs, purification cotton swabs are...
View detailsThese collection cards are treated with a proprietary chemistry intended to prevent environmentally induced degradation. They allow long term preservation of DNA from biological fluids stored at...
View detailsEfficient collection and release of samples, the best tool for collecting in molecular diagnostics, sterile materials
View detailsThe CHG IPA applicator is a single-use sterile product containing Chlorhexidine gluconate (CHG) at 2% w/w and isopropyl alcohol (IPA) at 70% v/v.
View detailsWe value your privacy We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
Our Privacy Policy