China Viral Transport Medium Tube: Swabs & VTM Supplier
4456The 10-mix-1 virus transport medium has become an effective tool for covid-19 PCR assays because it improves the efficiency of sampling and detecti...
View detailsSearch the whole station Pandemic Supply
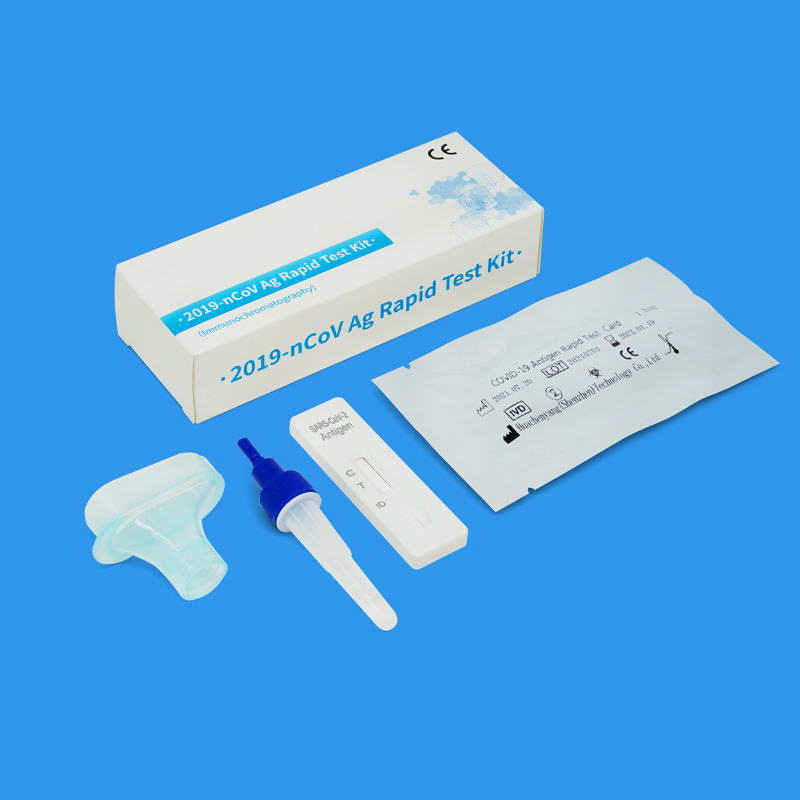
On 2022-5-24, COVID-19 Ag rapid self-test kit (Colloidal Gold) developed by Huachenyang (Shenzhen) Tech Co., Ltd. was awarded the EU CE 2934 certificate. (Due to the imminent implementation of IVDR and the transition policy, the IVDD CE certificate for this product is valid until May 2025.)
The Covid-19 Ag rapid test kit is divided into a professional version (to be operated by professionals) and self-test version (available for personal use). However, the EU market access requirements for these two are different. The manufacturer only needs to self-declare the conformity (DoC) of the product and submit the technical data to the European representative for filing in order to sell to the EU market. The Covid-19 Ag Rapid Self-Test Kit belongs to the Self-Test category, which requires the intervention of a third-party Notified Body (NB body) that meets the EU’s requirements to conduct a rigorous technical review and test of the product to prove that the product is safe and clinically reliable, and meets international technical specifications before a CE certificate can be issued. After the certificate is issued by the NB agency, the product can be circulated and sold in the EU according to the self-test product category.

Since then, Huachenyang’s COVID-19 Ag rapid self-test kit is available for sale in EU countries and countries that recognize the EU CE marking. Individuals can purchase and use it in pharmacies or supermarkets, which will help people to do self-monitoring and self-management in the fight against Covid-19.
Huachenyang’s Covid-19 Ag rapid self-test kit has not only obtained the EU CE access, but previously, this product has been approved by ThaiFDA (T6500115) on March 5 this year, which all prove that Huachenyang’s in vitro diagnostic reagent R&D and production strength has been internationally It also proves that Huachenyang’s international competitiveness is increasing.

COVID-19 has still not been eradicated from humans and the virus mutates frequently. COVID-19 antigen test can be used as a supplementary diagnosis and is an important tool for epidemic prevention and control.
The COVID-19 Ag rapid self-test kit, which received the EU CE certification, is a rapid test product that makes it very easy to use, and it has the following main features.
Huachenyang (Shenzhen) Tech Co., Ltd. was founded in 2008 and has been dedicated to the life and health industry for many years, continuously showing its brand strength of independent research and development and innovative development to the world. Huachenyang (Shenzhen) Tech Co., Ltd. will actively respond to the evolving needs of the epidemic and continue to provide higher quality products and services to our global customers and partners to better contribute to the fight against the epidemic!
E-mail: info@huachenyang.com
Tel: 0755-27393226 / 0755-29605332
Website: https://www.chenyanglobal.com
“HCY, Health care for you” is our forever mission. We dedicate to offering safe & reliable products and medical services with our global creditable partners. HCY has already supplied to WHO, MAYO clinic, MGI, DDC, Yale University, Qorvo, Quanterix, Thomas Scientific, SD biosensor, Cardinal Health, Cleveland Clinic, Mars Petcare & LumiraDx, etc. in the past years.
The 10-mix-1 virus transport medium has become an effective tool for covid-19 PCR assays because it improves the efficiency of sampling and detecti...
View detailsHow to use iClean COVID-19 Antigen Rapid Test Kit? Here is a video tutorial.
View detailsThe COVID-19 antigen rapid test kit is designed to identify the SARS-CoV-2 nucleocapsid antigen.
View detailsLollipop rapid saliva test kit is an immunochromatographic membrane assay for detecting SARS-CoV-2 N protein antigen in human saliva samples.
View detailsWe value your privacy We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
Our Privacy Policy